
RIKEN (Ryoji Noyori, President) has suggested that dementia, due to the accumulation of not only mutant tau protein, but also in naive tau protein. This has been achieved by the joint research of Toyama University, Gunma University, and University of Florida, including Hirotaka Onoe, Team Leader of Functional Probe Research Laboratory, RIKEN Center for Molecular Imaging Science (Yasuyoshi Watanabe, Director), Hiroshi Mizuma, researcher, et al., Akihiko Takashima, Team Leader of the Lab for Alzheimer's Disease, RIKEN Brain Science Institute (Susumu Tonegawa, Director), Yumiko Motoi, associate professor, and Yasunori Kambe, assistant professor of Department of Neurology, Juntendo University Hospital.
Tau protein is required for stabilizing consecutive axonal microtubules in neurons, which comprise the neuronal network. However, when the abnormal hyperphosphorylated tau protein is formed the insoluble aggregation, which lead to the formation of neurofibrillary tangles and, subsequently, neuronal death. Such intracellular tau deposit-mediated neurodegeneration are called "tauopathies," Alzheimer's disease (AD) and frontotemporal lobar degeneration (FTLD). Numerous studies have been carried out using transgenic mice by expressing various mutant tau proteins to clarify the mechanism by which pathogenesis of tauopathy, but it is unclear how the mutant tau protein are closely related to the neurodegeneration.
In this study, to clarify whether neuronal accumulated naïve tau protein induces neurodegeneration, the research group has developed a tauopathy model mouse (Tg601) by overexpressing a wild-human tau protein in neuron. Interestingly, the Tg601 mouse displayed spatial learning deficit and impaired anxiety in the aged period. Since the behavioral abnormalities were thought to be caused by the neural dysfunction correspond to the frontal cortical region in human brain, the research group has conducted in vivo brain imaging using positron emission tomography (PET) with 18Fluorine-labeled fluorodeoxyglucose in the Tg601 mouse under conscious condition, The Tg601 mouse showed low neural activities only in the nucleus accumbens, which is related to the emotional behaviors such as motivation and fear. Furthermore, it was observed the different forms of phosphorylated tau protein in the striatal region, including nucleus accumbens with aging by histological study.
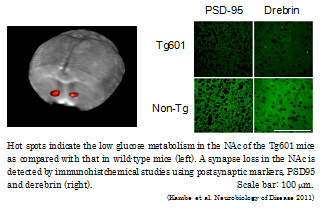
Taken together, these findings indicated that the wild-type human tau protein induces neurodegenerative disease in aging is similar to the symptom of FTLD and that the neurodegeneration caused by naïve tau protein is strongly related to the presence of phosphorylated tau, it is expected that Tg601 mouse is useful for understanding the pathogenetic mechanisms of tauopathy. The achievements of this study were published in the Neurobiology of Disease. (June Issue)
