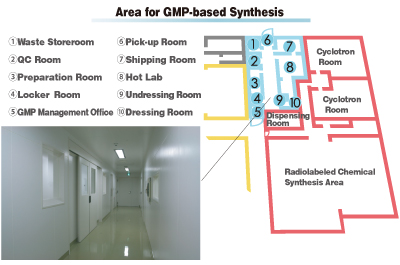
After molecular probes that are identified as drug candidates have been confirmed with regard to safety and efficacy through animal experiments, the next step is to move on to clinical studies in humans. The national government requires that PET probes to be administered to humans be produced in line with manufacturing and quality control regulations (called Good Manufacturing Practice, or GMP). CMIS includes an area for GMP-based synthesis that complies with these regulations. PET probes synthesized in this area provide a safe, stable supply for medical institutions engaged in clinical research, translating the results of basic research carried out on animals into clinical research.
